Clinical biochemistry
3. Overview of the most common laboratory tests
3.1. Determination of Na, K, Cl
Reference range
S_Na 137 - 145 mmol/L
S_K 3,6 - 4,8 mmol/L
S_Cl 98 - 109 mmol/L
S_osmolality 275 - 295 mmol/kg
U_ osmolality (random sample) 300 - 900 mmol/kg
It is a basic test, the results of which are used for most diseases - from acid-base balance disorders to kidney and liver diseases to hormonal disorders. Sodium metabolism is closely related to water metabolism and the maintenance of an effective circulating volume. Total body water (CTV) accounts for about 70% of body weight in men and 60% in women. For example, for a 70 kg man, we assume a CTV of 49 L. Intracellular fluid (ICT) makes up 2/3 of CTV, the rest is reserved for extracellular fluid (ECT). Plasma makes up ¼ of ECT, the remaining ¾ is occupied by interstitial fluid. The composition of plasma and interstitial fluid is similar, only in the interstitial fluid proteins are almost absent (they are replaced by chlorides). The main extracellular cation is sodium, while chloride and bicarbonate predominate among the anions. The main intracellular cation is potassium, phosphate and protein predominate among the anions. Ion and water transfers between the ICT and ECT are involved in many ionic imbalances. The difference in concentrations of substances that do not freely pass between ICT and ECT is crucial for water transfers between ICT and ECT: these are cations and anions and glucose. These substances determine the so-called effective osmolality of the ECT, and we estimate it as 2 x Na+ + glucose (we multiply the concentration of Na+ by 2 to include the corresponding anions - e.g. Cl- and HCO3-). In contrast, substances such as urea or ethanol pass freely between ICT and ECT - changes in their concentration do not lead to water shifts.
The major system that regulates

ADH is produced in the hypothalamus and secretion is primarily controlled by plasma osmolality, which is detected by osmoreceptors in the hypothalamus. As the effective plasma osmolality increases, ADH secretion increases - the body attempts to compensate for the high plasma osmolality by greater renal water absorption. However, there are also non-osmotic stimuli to increase ADH production - e.g. hypovolaemia, nausea and vomiting, some drugs. These may be important in the pathophysiology of e.g. hyponatremia. The recruitment of ADH to V2 receptors in the collecting duct of the nephron results in the "opening" of water channels (aquaporins) and increased water absorption.
Hyponatremia is a blood sodium concentration below 135 mmol/L. Most often, the osmolality is also adequately reduced (hypoosmolar hyponatremia). However, the cause is renal (e.g., administration of thiazide diuretics) or extrarenal (e.g., GIT secretions or sweating) loss of sodium, often as a result of hypoosmolar fluid loss -> increased ADH secretion due to hypovolaemia -> water replenishment by drinking and relative excess water. Adjunct hyponatremia is the cause of primary polydipsia (water intoxication). The high osmolality in hyponatremia (hyperosmolar hyponatremia) is found especially in hyperglycemia, e.g., in a decompensated diabetic patient - high glucose concentration in ECT will lead to transfer of water from ICT to ECT and consequent dilution of Na+ concentration in plasma. If hyponatremia is accompanied by normal serum osmolality, this is an error in sodium determination - high dilution during Na+ determination with ion-selective electrodes on automated analyzers will cause falsely low Na+ concentrations in samples with extremely elevated lipid (chylose serum) or protein (e.g., multiple myeloma) concentrations. The basic laboratory diagnostic approach to hypoosmolar hyponatremia is based on urine osmolality and sodium values (Figure 7).
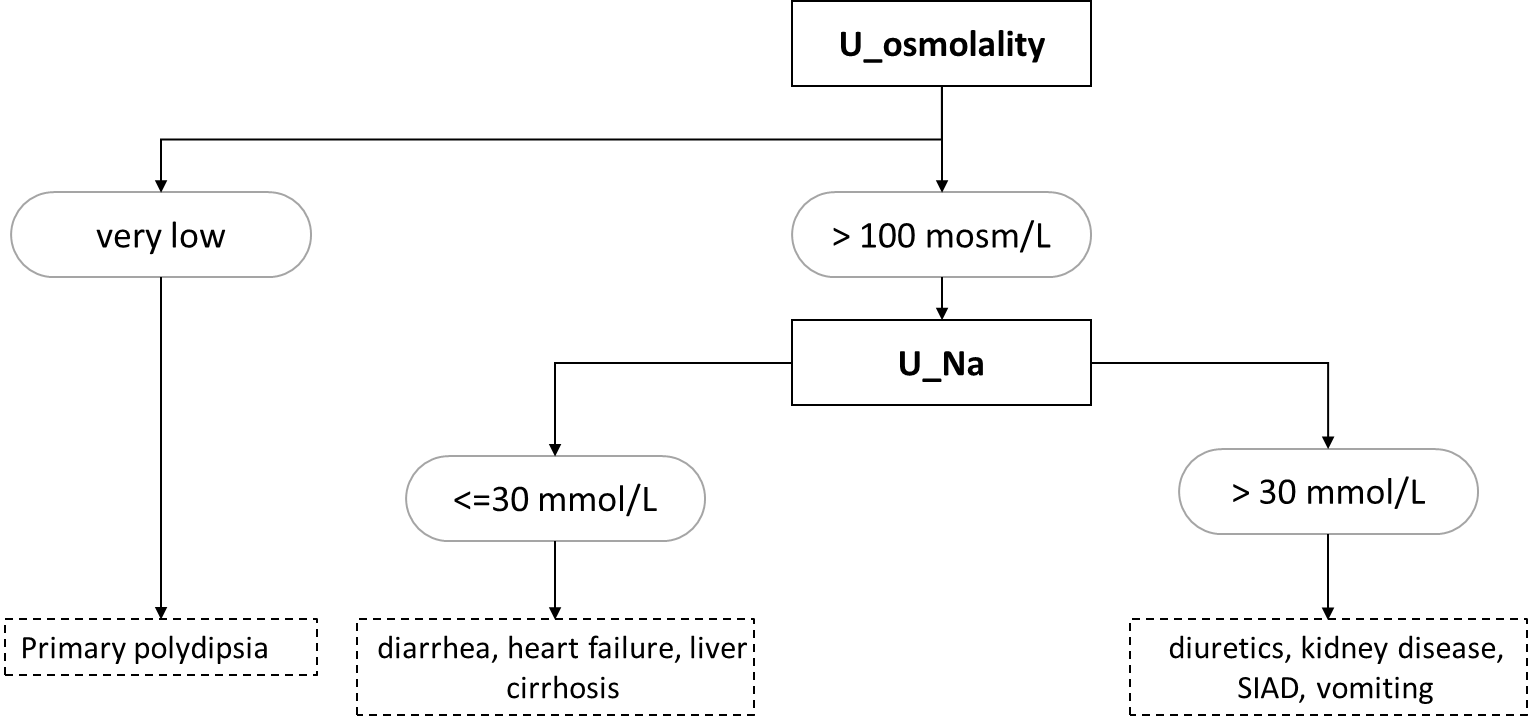
Hypernatremia is a blood sodium concentration above 145 mmol/L. It is most often caused by loss of pure water or hypoosmolar fluid that is not replenished by drinking. This includes bedridden patients who, for example, lose fluids during fever or polyuria and cannot replenish them by drinking without the help of nursing staff. Alternatively, self-sufficient patients who do not have access to drinking water. The basic diagnostic approach to causes of hypernatremia is based on measurement of urine osmolality - if very low (below 300 mosm/kg), the cause of hypernatremia is diabetes insipidus; if very high (above 600 mosm/kg), the cause is thirst or fluid loss through the gastrointestinal tract or sweat.
Hypokalemia is a concentration of potassium in the blood below 3 mmol/L. The most common causes are loss through the gastrointestinal tract by vomiting or diarrhea, administration of crank diuretics, primary hyperaldosteronism, and alkalemia. Alkalemia leads to transfer of K+ into cells, whereas acidemia leads to transfer of K+ out of cells. In extrarenal losses, the concentration of K+ in urine is very low.
Hyperkalemia is a concentration of potassium in the blood above 5 mmol/L. The most common causes are reduced renal excretion in renal failure (see below) or administration of hyperkalemic therapy and transfer of K+ from the ICT in acidemia. Examples of hyperkalemic drugs include ACEIs, ARBs, potassium-sparing diuretics (spironolactone, triamterene).
The assessment of chloride concentration in the blood is essential for the interpretation of the acid-base balance and will be discussed there.