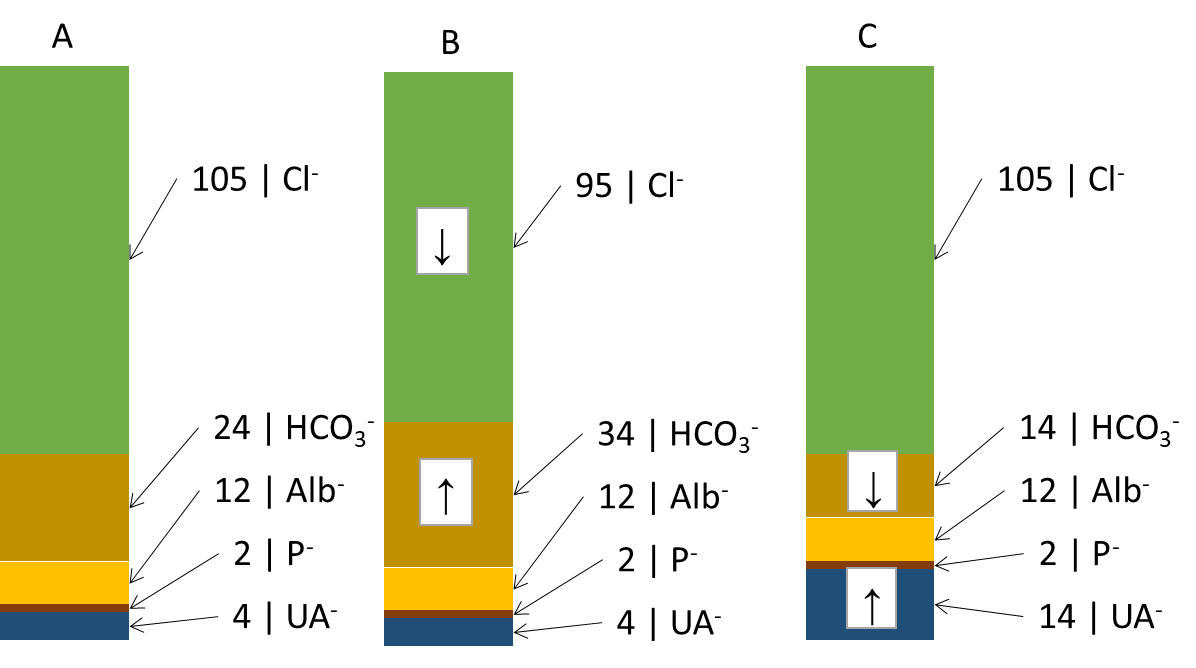Clinical biochemistry
3. Overview of the most common laboratory tests
3.2. Introduction to acid-base balance disorders
Reference range
pH 7.36 - 7.44
pCO2 4,8 - 5,8 kPa
HCO3- 22 - 26 mmol/L
BE -2.5 - 2.5 mmol/L
AG 14 - 18 mmol/L
The homeostasis of hydrogen ions is essential for the maintenance of optimal enzyme function and protein ionization; significant changes in the concentration of H+ bind e.g. active cellular transporters (e.g. Na+/K+ ATPase) and energy production (enzymes of glycolysis and lipolysis). The terms acidemia and alkalemia are used for increased and decreased H+ concentrations, respectively. For acid-base balance (ABR) disorders, the term alkalosis a acidosis is reserved, which reflects the whole pathophysiological process with its cause and compensation, in addition to the H+ concentration itself. The organism tries to maintain the optimal concentration of H+ in the following ways:
- buffers - generally they are salts of weak acids, which can bind H+ in case of acidemia, and release H+ in case of alkalemia. The most important buffer of ECT is hydrogen carbonate (HCO3-); in ICT, the most abundant is phosphate, proteinate and in erythrocyte hemoglobin. At elevated H+ loading, buffering is distributed between ICT and ECT with a slight predominance of buffering capacity in the ICT. These are fast reactions (on the order of seconds), but can take up to hours to propagate between ICT and ECT.
- Organ Compensation - the most important organs that compensate for ABR disorders are the kidneys and lungs. The kidneys compensate for excess H+ in acidosis by excreting H+ and Cl- in the urine and by newly formed HCO3-. Most of the H+ is excreted by urinary buffers: phosphate and ammonium. Ammonium buffer is more significant in compensations, it can increase its excretion capacity considerably (5 times). The principle of this compensation is a gradual (over 3 to 5 days) increase in ammonia production from glutamine in cells of the proximal tubule. Ammonia is then excreted in the form of ammonium chloride in the urine. Or in the case of acidosis caused by one of the non-resorbable acids - e.g. ketoacidosis or acidosis in ethylene glycol poisoning - the ammonium cation binds to the anion of the corresponding acid. Adaptation of the kidney to alkalosis is much less effective or counterproductive. The most common causes of metabolic alkalosis (vomiting and diuretics) lead to activation of the RAAS, to hypochloremia and intracellular acidosis. RAAS activation causes Na+ absorption, with which HCO3- is partially absorbed. Hypochloremia prevents the excretion of HCO3- by the passive tubular antiport HCO3-/Cl-. Compensatory hypoventilation leads to hypercapnia, which causes intracellular acidosis to which tubular cells respond by secreting H+. These compensatory events in the most common causes of metabolic alkalosis thus result in paradoxical further secretion of H+ and retention of HCO3-. The lungs compensate for metabolic acidosis by hyperventilation = lowering the partial pressure of CO2 (pCO2) in the blood and for metabolic alkalosis by hypoventilation = increasing the pCO2 in the blood. However, hypoventilation is limited by hypoxia and is not very effective.
The essential laboratory parameters for the assessment of ABR in blood are H+, pCO2, Na+, K+, Cl-. We traditionally express H+ as the negative decadic logarithm of the activity of H+, pH. It is therefore a logarithmic scale to which we are not normally accustomed, and we have to reckon with the fact that a small numerical change means a large change in concentration. From H+ and pCO2 we calculate HCO3using the relationship:
It is also clear that the resulting concentration of H+ depends on the ratio between H2CO3 and HCO3-, not on their absolute concentration. The concentration of H2CO3 corresponds to pCO2, in the above relation we can use pCO2 after conversion by the solubility coefficient.
Another useful derived relationship is thebase excess extracellular fluid (BEECT). It is a parameter defined simplistically as the amount of strong acid that must be added to the model fluid corresponding to the ECT to return the pH to normal (assuming pCO2 is normal). BE attempts to summarize the effect of the non-respiratory component (bicarbonate and other buffers - mainly hemoglobin) on ABR. Changes in the concentration of hemoglobin are rather negligible from the point of view of buffering capacity, BEECT thus practically expresses changes in HCO3- caused by metabolic (non-respiratory) influences. Negative values indicate an excess of acids - metabolic acidosis, positive values a deficiency of acids - metabolic alkalosis. However, BEECT does not inform us in any way about the cause of the metabolic disorder and is a summative reflection of all metabolic influences: we therefore do not detect combined disorders. These shortcomings are improved by electroneutrality theory, which very simplistically states that the sum of all positive and negative charges in the ICT and ECT is zero. Thus, there is an equal number of anions and cations. Therefore, for example, an increase in one anion must lead to a decrease in another or an increase in a cation. We are most interested in the anion column, where we find the main determinants of the ABR state: chloride, bicarbonate and unmeasured anions. In Figure ... we see (A) normal anion concentrations in plasma, (B) an example where the patient loses chloride - by vomiting or after administration of furosemide; another anion - HCO3- (provided by the kidneys and other mechanisms) - must rise to compensate. If the production of relatively strong acids, such as β-hydroxybutyric acid or lactic acid (C), rises, the increased H+ load is buffered by HCO3- (and other buffers) and the concentration of HCO3-decreases. However, the situation in Figure ... is very simplistic - the H+ load is distributed between ICT and ECT, and the effect on the decrease in HCO3- and BEECT may not be exactly 1:1. In any case, once equilibrium is established, the equilibration of the ratios between ICT and ECT reflects BEECT all relevant metabolic (non-respiratory) influences. Chloride on the one hand and unmeasured anions on the other hand determine the magnitude and direction of BEECT; if we find the cause of the change in Cl- and unmeasured anions, we also reveal the cause of the ABR disturbance.
In Figure 8 we did not account for changes in cations. These are also important for ABR, especially changes in Na+ relative to changes in Cl-. If both ions change proportionally, there is no reason to change the concentration of HCO3-. If, however, Cl-
Clcor = Cl- x 140/Na+
Unmeasured anions are simplified by the calculation of the anion gap (AG)
AG = (Na+ + K+) - (HCO3- + Cl-)
The formula does not take into account some plasma cations (Ca2+, Mg2+) and anions (albumin charge, phosphate charge, unmeasured anions) in the plasma and expresses just their difference (12 + 2 + 4 on the side of anions see Figure 8 and subtract 1.5 + 1.5 for unmeasured cations, the result is 15 mmol/L). In unmeasured anions we count ketoacid anions in ketoacidosis, lactate in lactic acidosis, or acid anions formed during the metabolism of ethylene glycol (oxalate), ethanol (acetate), and methanol (formate) or salicylate. As mentioned above, unmeasured anions are, together with chloride, the main factors determining the concentration of HCO3- in plasma. Thus, AG reveals important causes of metabolic acidosis. It is also clear that most AG is physiologically albumin - with significant changes in albumin concentration, the AG calculation may be biased and may not reveal the presence of unmeasured anions. It is therefore advisable to correct the AG for the actual albumin concentration. We start from the simplified assumption that the albumin charge in ¼ mmol/L corresponds to ¼ of the albumin concentration in ¼ g/L. Thus, if albumin drops from 40 g/L to 20 g/L, the albumin charge drops from 10 mmol/L to 5 mmol/L.
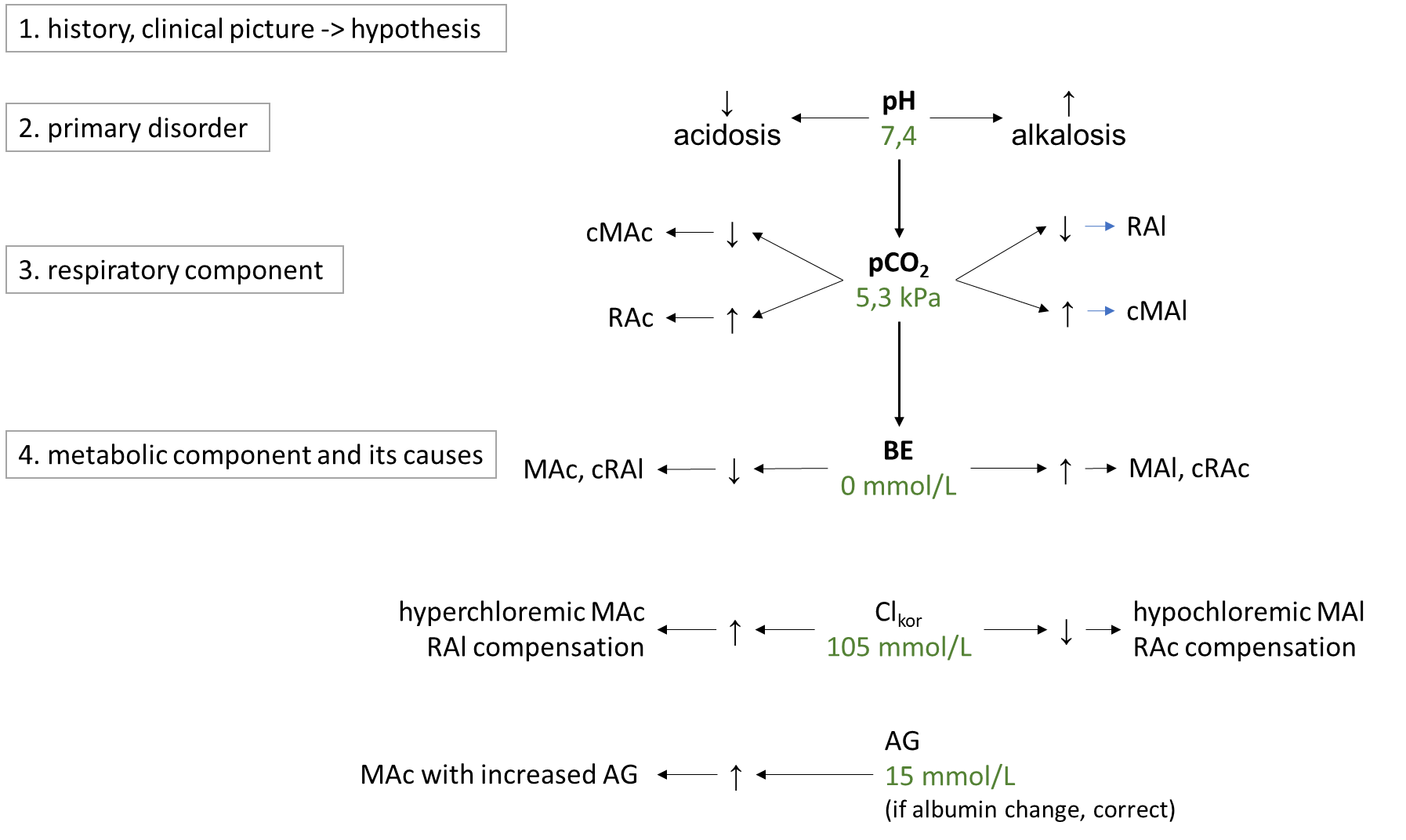
ABR disorders are divided into respiratory and metabolic disorders.Respiratory disorders are primarily caused by hyperventilation (respiratory alkalosis, low pCO2; RAl) or hypoventilation (respiratory acidosis, high pCO2; RAc).The cause of RAl is e.g. hyperventilation during emotional tension, pain, discomfort, in the early stages of hypoxia, in sepsis or irritation of the respiratory centre by salicylates or in CNS pathology. Causes of RAc include respiratory failure, chronic obstructive bronchopulmonary disease, or pharmacologic attenuation of the respiratory center by opiates. Metabolic disorders are primarily usually caused by changes in chloride and in unmeasured anions, which are reflected in bicarbonate concentrations. In simple terms, the metabolic component is expressed by BEECT - low indicates metabolic acidosis, MAc, high metabolic alkalosis, MAl. The cause of MAc may be e.g. A rise in chloride (hyperchloremic MAc) after saline administration, in diarrhea (losses of HCO3- through diarrhea are replaced in the anion column by chloride), or in ureterosigmoidostomy (in the colon, Cl- is absorbed from the urine). Another group of causes of MAc are acidosis due to increased production of organic acids in ketoacidosis, lactic acidosis, ethanol, methanol, ethylene glycol or salicylates and paracetamol poisoning. All of these acidoses are caused by an increase in unmeasured anions, so they are acidoses with increased AG (normochloremic). The most common cause of MAl is loss of chloride during vomiting or during administration of furosemide (hypochloremic MAl) ev. MAl caused by increased mineralocorticoid exposure or severe hypokalemia. A basic overview of how to approach the evaluation of ABR disorders is shown in Figure 9. First, we estimate the likelihood of the presence of ABR based on the history and clinical status and hypothesize what disorders come into consideration in the case. Then, in successive steps, we evaluate laboratory parameters: pH to determine the primary ABR-> disorder; pCO2 to elucidate the influence of the respiratory component, and finally BEECT to determine the summary metabolic component. For the metabolic component, we then determine the influence of corrected chloride and AG.
Figure 9. Basic diagnostic approach to ABR disorders. More detailed description intext.